
FDA
FDA 21 CFR 1040.10 - Laser Product Performance Standards


Chromium excels in providing durable wear-resistant coatings that protect tools and components from harsh chemical environments and abrasion during extended industrial use
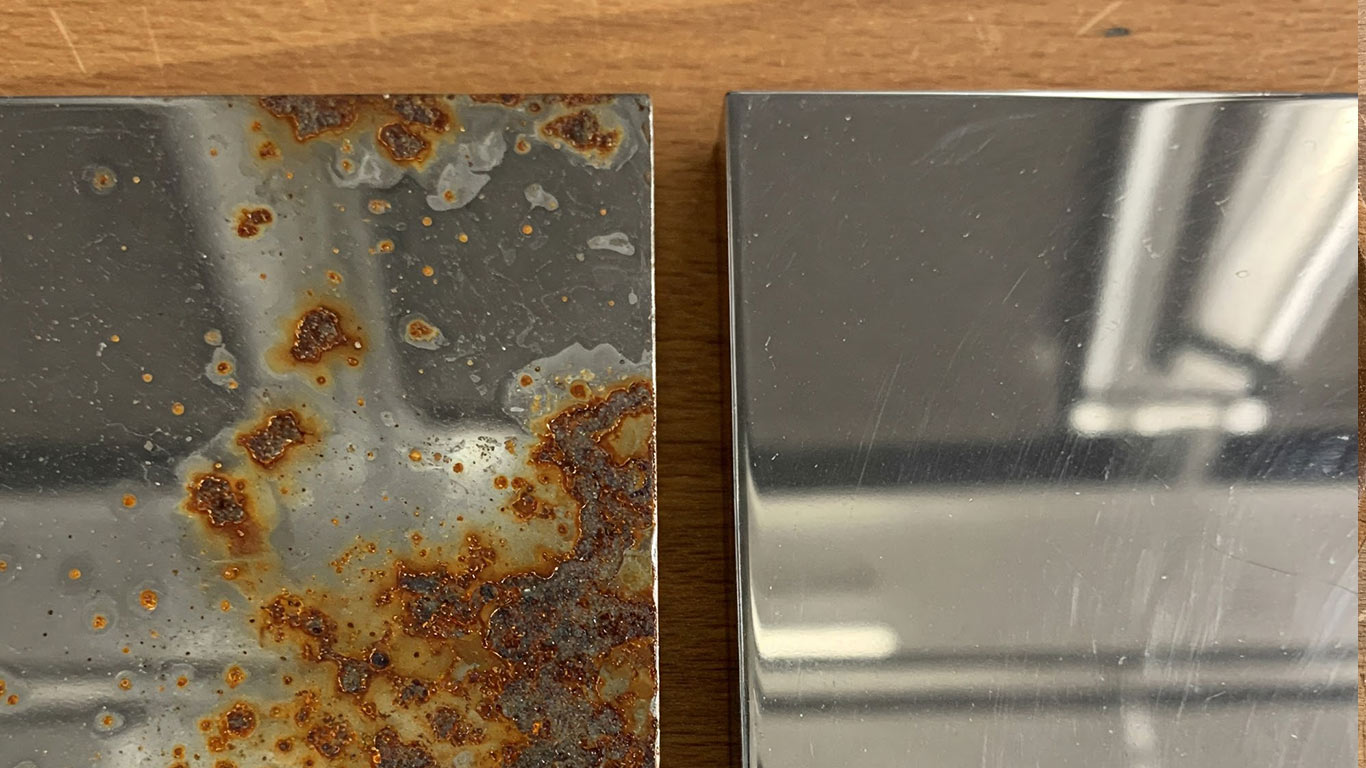
The contaminated chromium surface appears dull and uneven under magnification. Dark spots and fine particles cling to its texture, hiding the metal's natural shine. Scratches and residues make the whole area look rough and patchy.
Laser cleaning restores the chromium to a smooth, reflective state. Bright, uniform facets emerge without any clinging debris. The treated surface now gleams evenly, free of all visible imperfections.

FDA 21 CFR 1040.10 - Laser Product Performance Standards

ANSI Z136.1 - Safe Use of Lasers

IEC 60825 - Safety of Laser Products

OSHA 29 CFR 1926.95 - Personal Protective Equipment