


Mercury Spill Residue
Mercury contamination forms during industrial processes on metal surfaces, and residues deposit unevenly because vapor exposure creates thin films. Before cleaning, contamination spreads in irregular patterns, so laser application targets these layers carefully. Process removes mercury effectively from conductive materials, but challenges arise on porous substrates where residues bind tightly. During exposure, heat volatilizes the contaminant, yet incomplete removal occurs because particles re-deposit nearby. Surface exhibits stickiness after partial ablation, so multiple passes become necessary. In observations, mercury behaves differently on alloys compared to pure metals; it evaporates faster from the former, while buildup persists on the latter. Treatment achieves cleaner results on non-reactive surfaces, and analysis shows reduced residue adhesion post-process. Natural regional patterns influence formation in humid areas, where moisture aids deposition.
Produced Compounds
Affected Materials

Aluminum
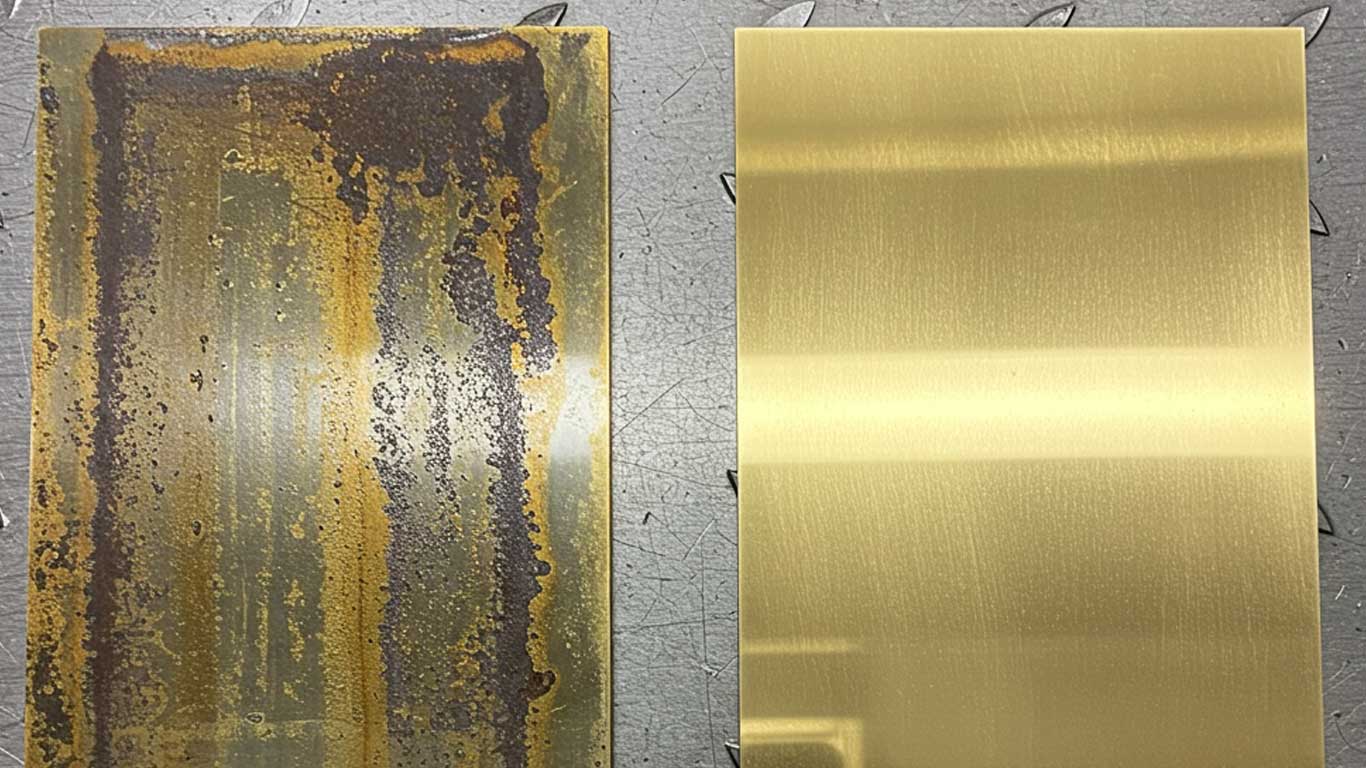
Brass

Brick

Bronze

Cast Iron
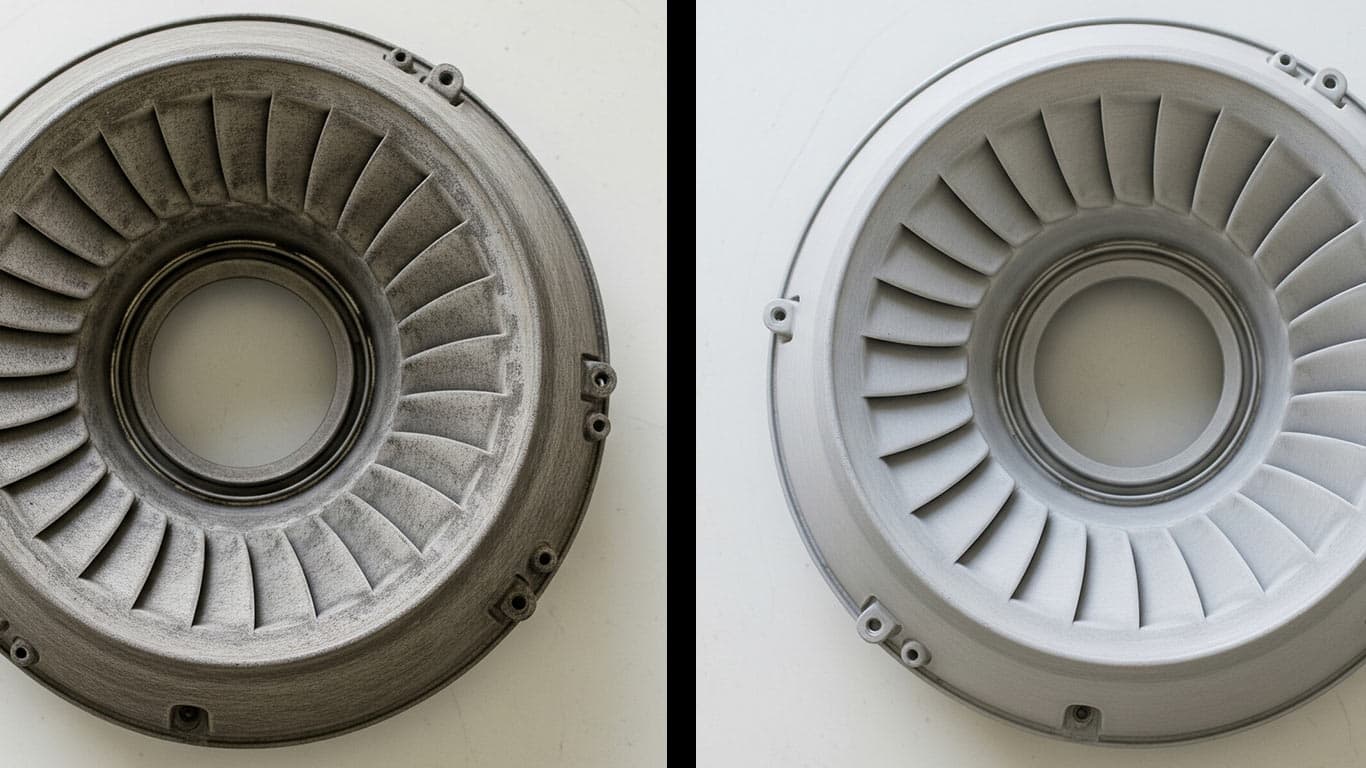
Ceramic Matrix Composites CMCs

Concrete

Copper

Granite

Iron

Limestone

Magnesium

Marble

Nickel

Porcelain

Sandstone

Slate

Stainless Steel

Steel

Terracotta

Titanium
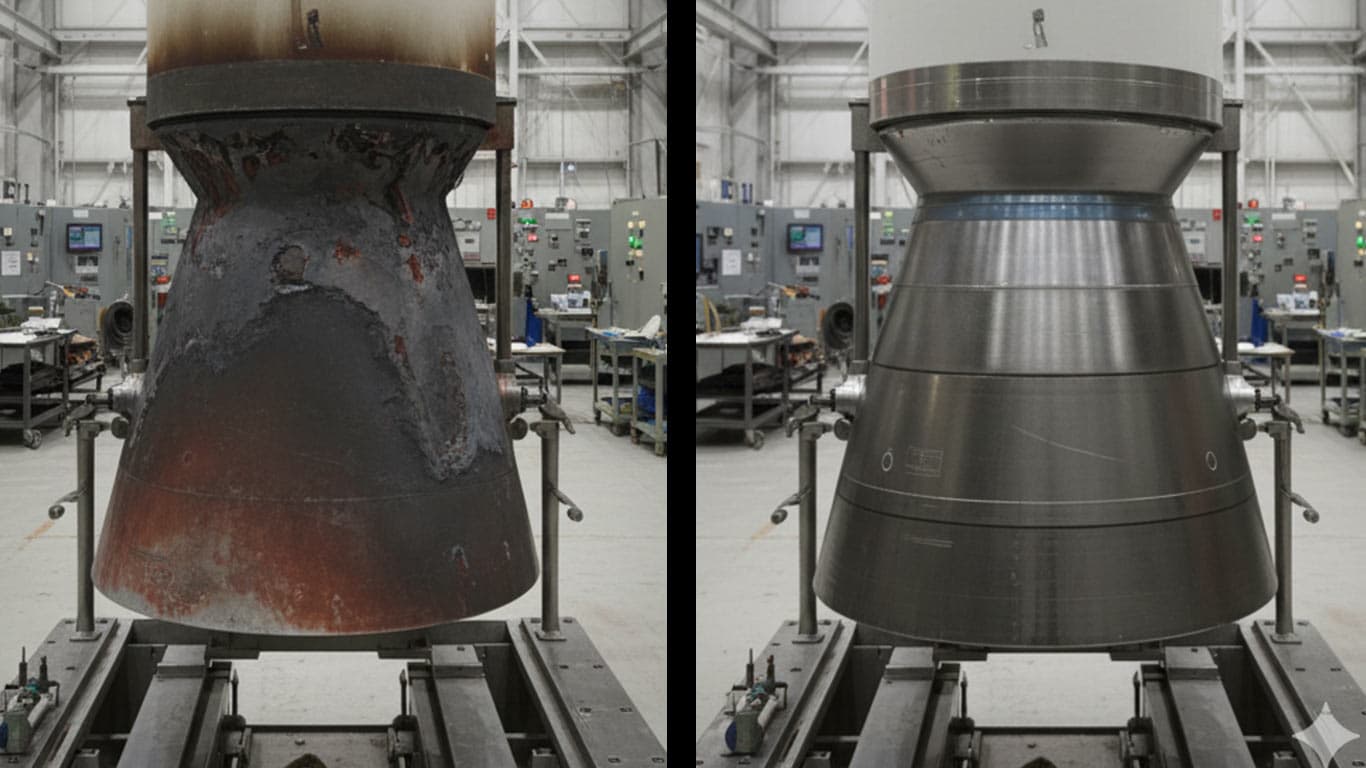
Titanium Carbide
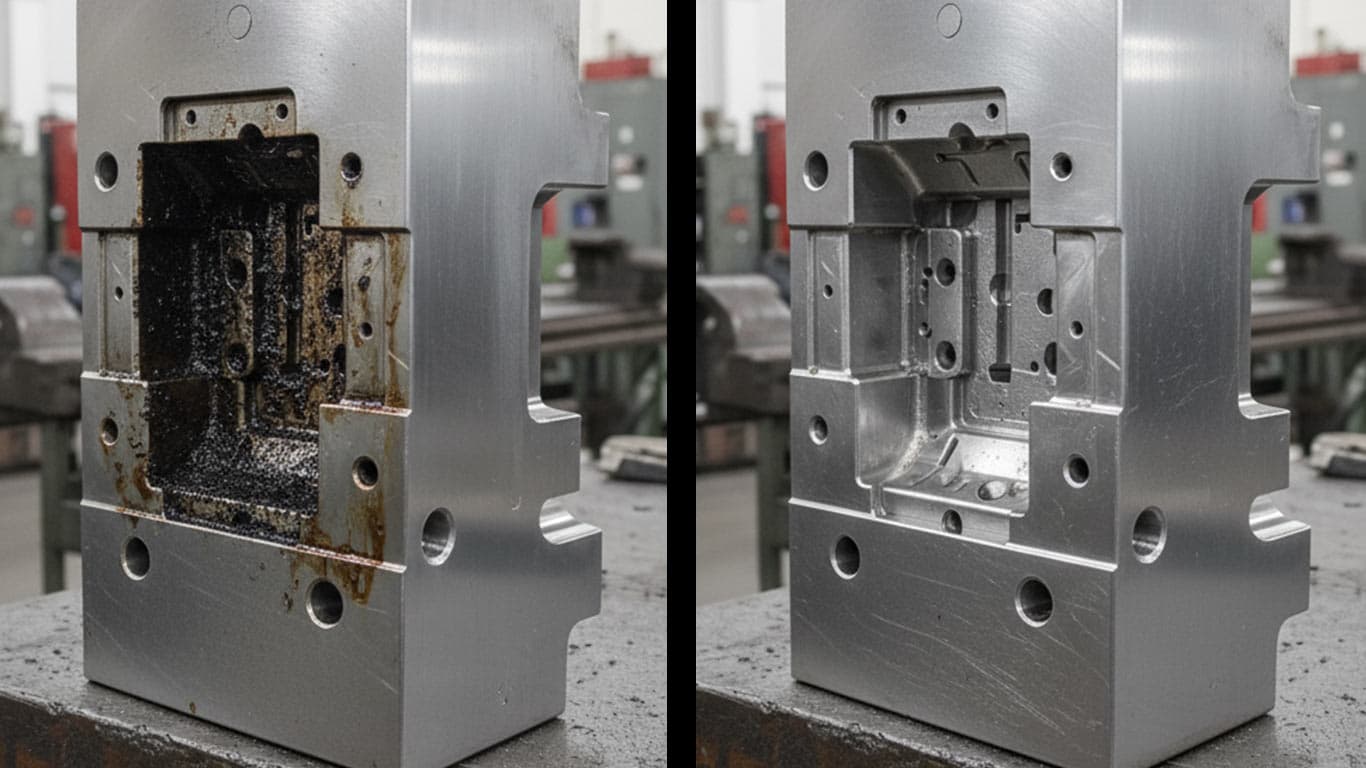
Tool Steel

Zinc
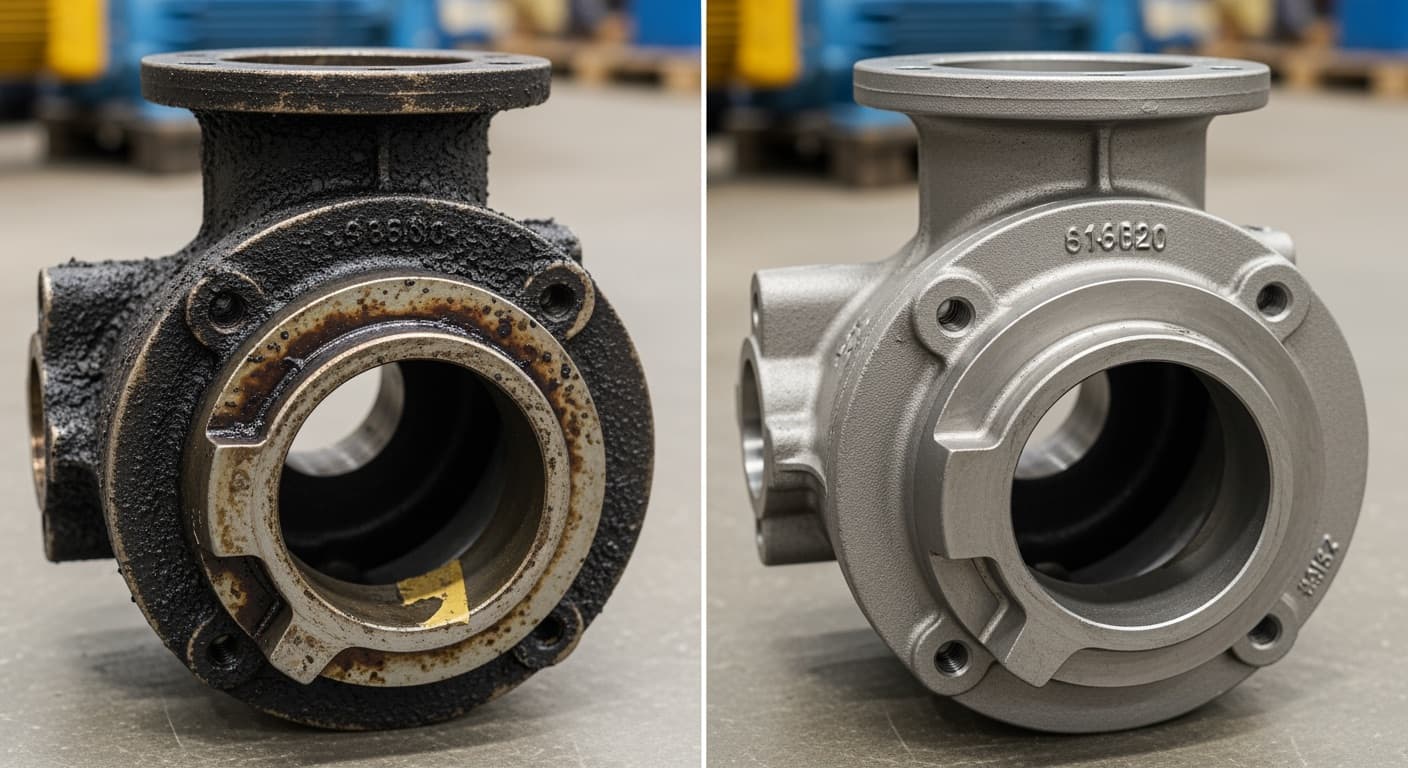
Titanium Alloy (Ti-6Al-4V)

Stainless Steel 316
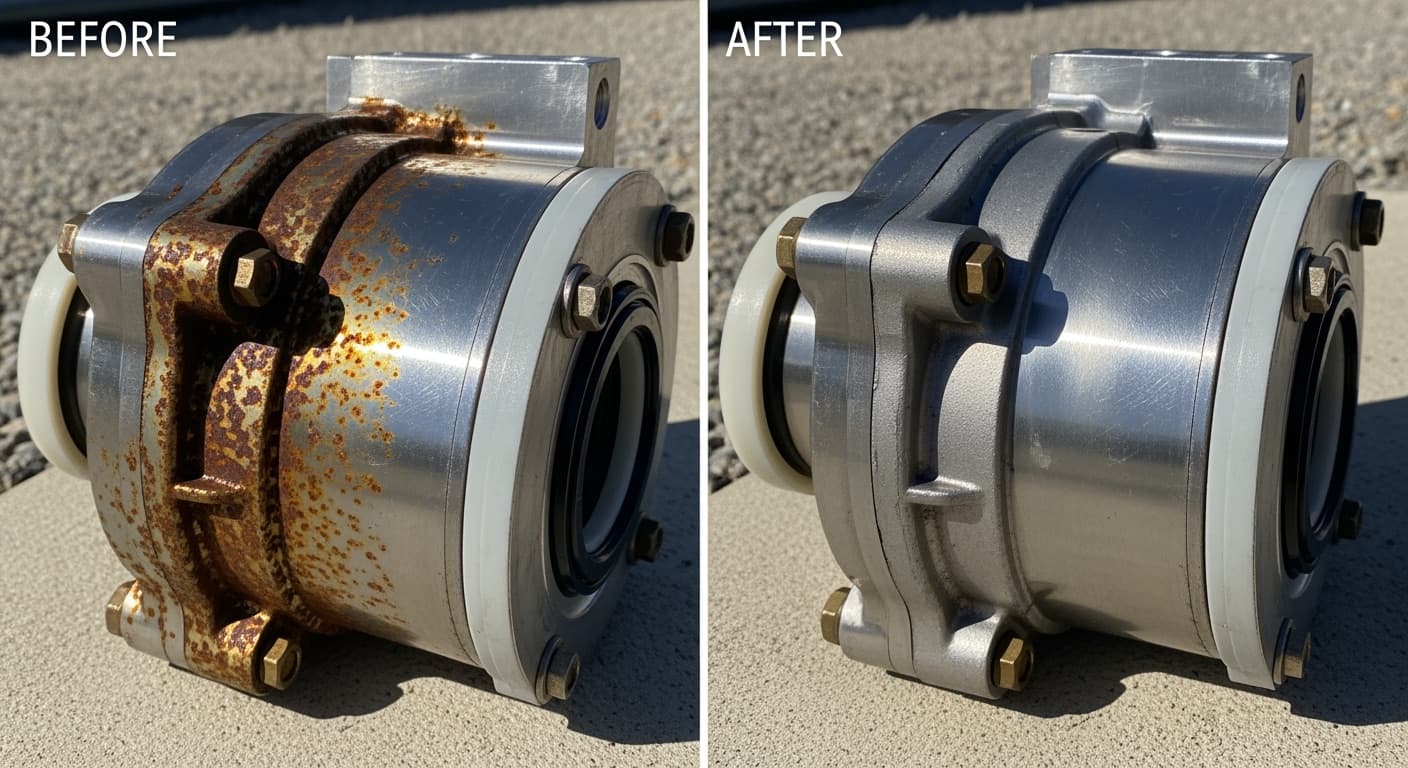
Stainless Steel 304

Aluminum Bronze

Aluminum Nitride
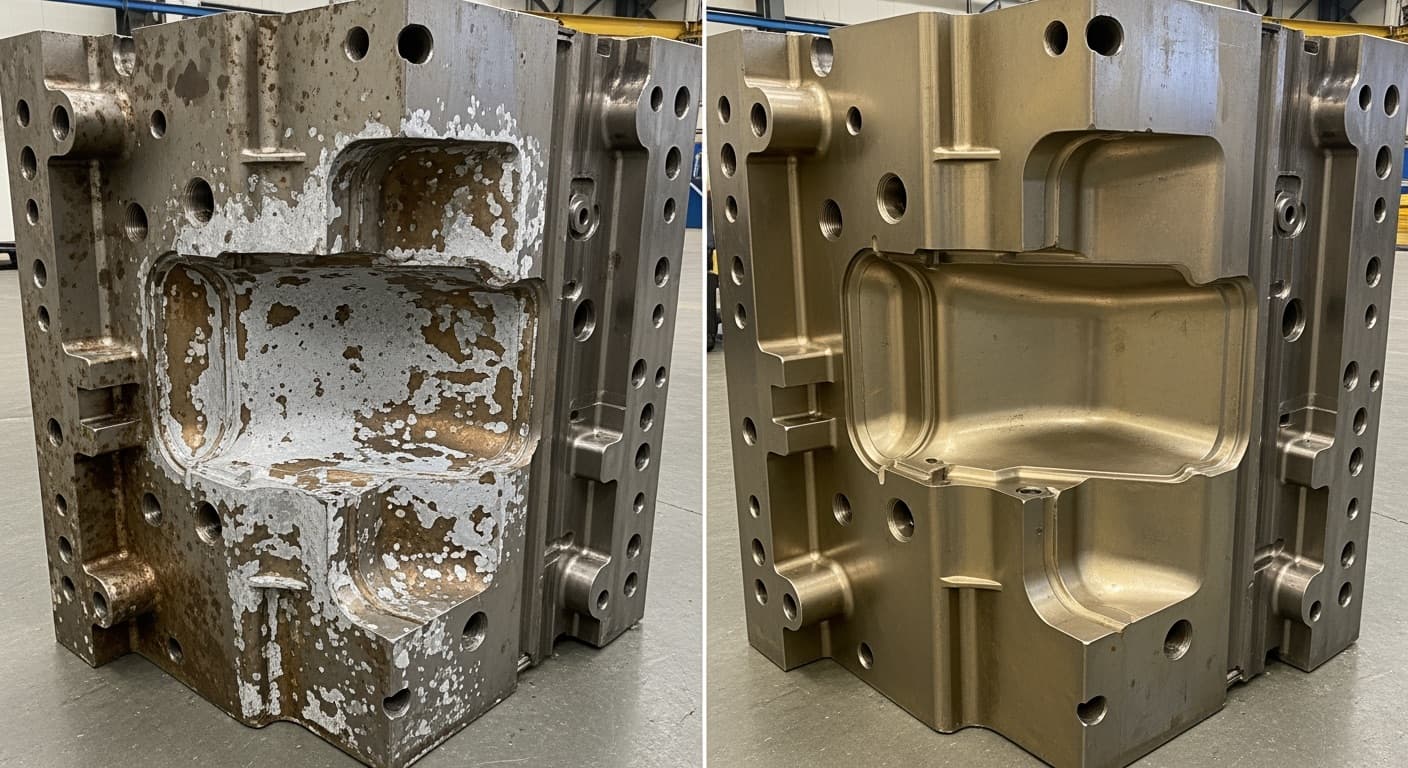
Titanium Nitride
Mercury Spill Residue Dataset
License: Creative Commons BY 4.0 • Free to use with attribution •Learn more

