


Pharmaceutical Drug Residue
Pharmaceutical-residue-contamination, it arises from sticky organic layers in drug production environments. This contamination forms unique patterns, such as bio-adhesive films on metal tools and crystalline deposits on glass vials, thus varies by substrate material. On plastics, residue spreads thinly and embeds deeply, while on stainless steel, it clings in irregular clusters. Removal challenges emerge from thermal sensitivity; laser cleaning applies energy, yet residue resists vaporization due to volatile components. After treatment, surface still shows traces in shadowed areas, so multiple passes become necessary. Material behaviors differ—metals conduct heat quickly and yield clean results, but polymers degrade easily and retain haze. Process demands precise pulse control, and thus enhances efficiency for sensitive substrates. Yi-Chun Lin, Ph.D., Taiwan
Produced Compounds
Affected Materials

Aluminum
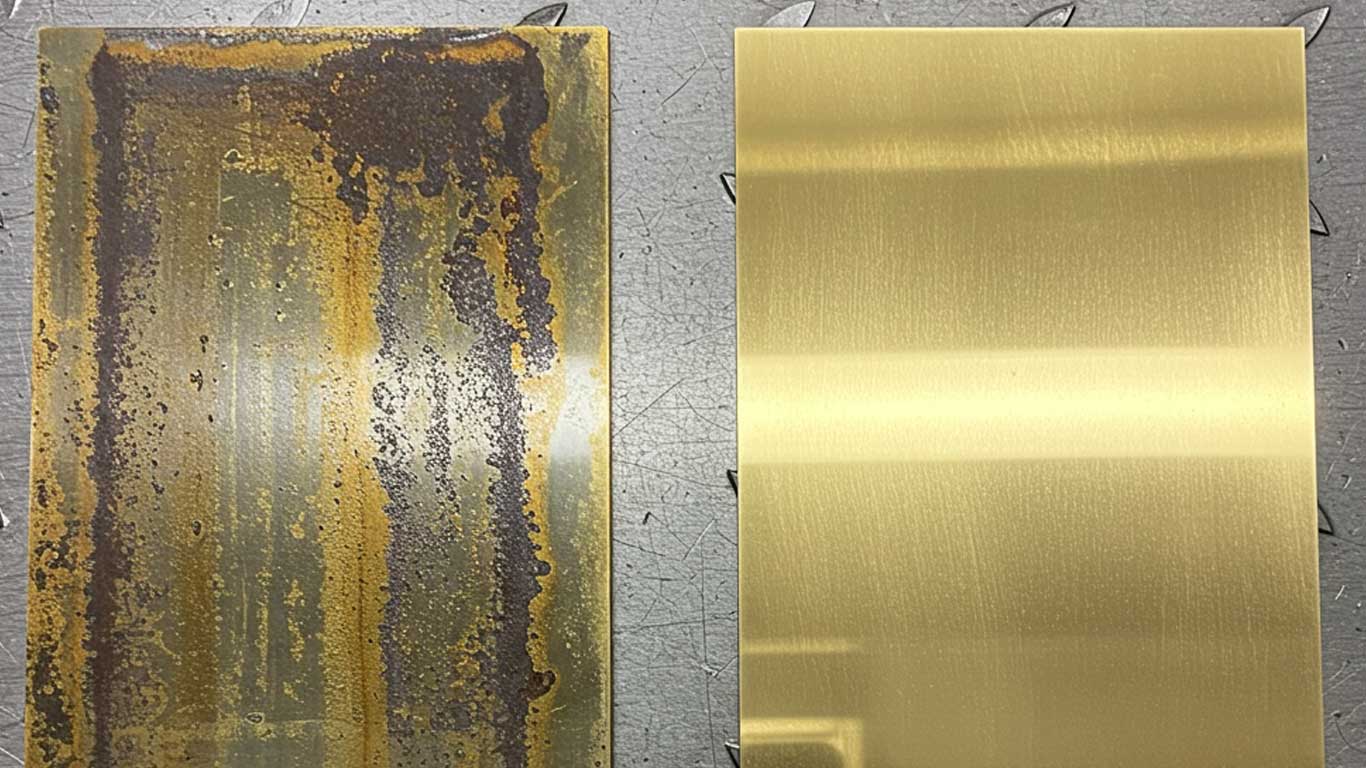
Brass

Brick

Bronze

Cast Iron
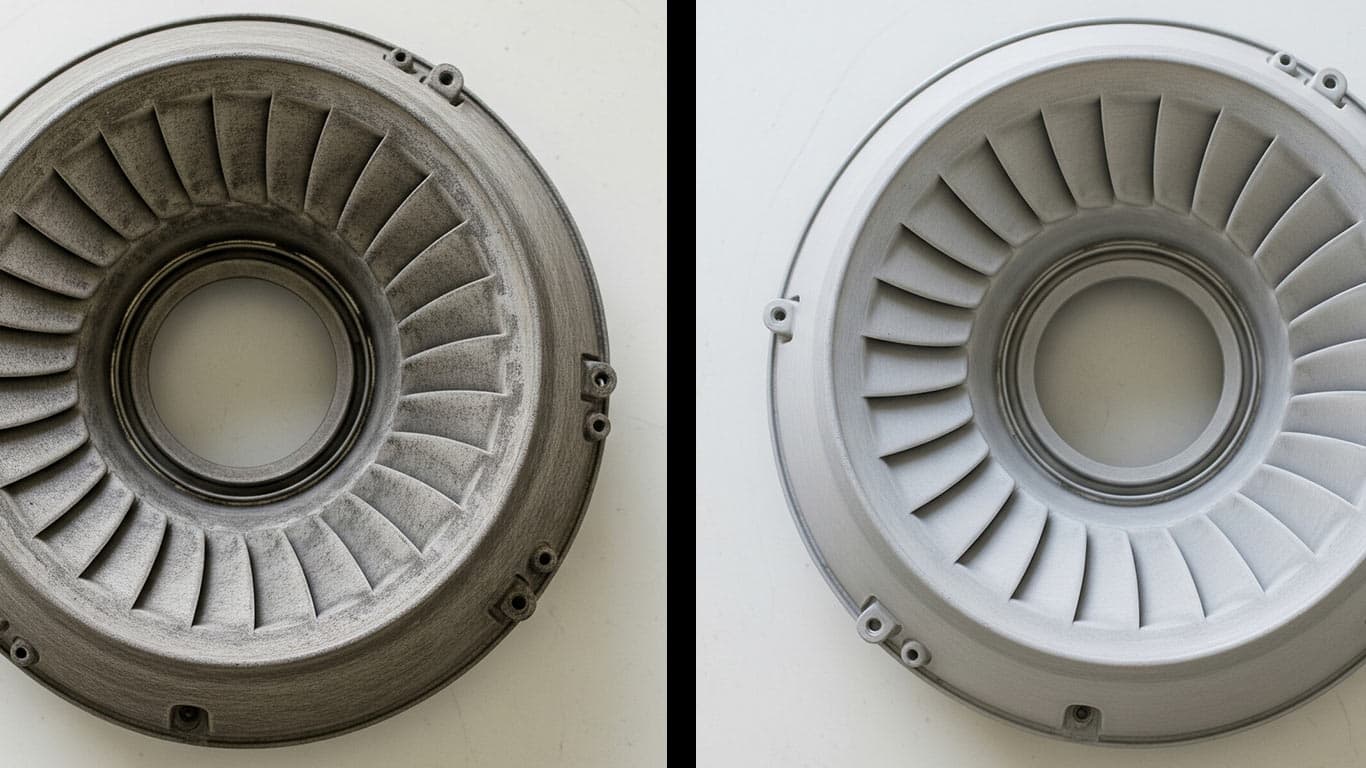
Ceramic Matrix Composites CMCs

Concrete

Copper

Granite

Iron

Limestone

Magnesium

Marble

Nickel

Porcelain

Sandstone

Slate

Stainless Steel

Steel

Terracotta

Titanium
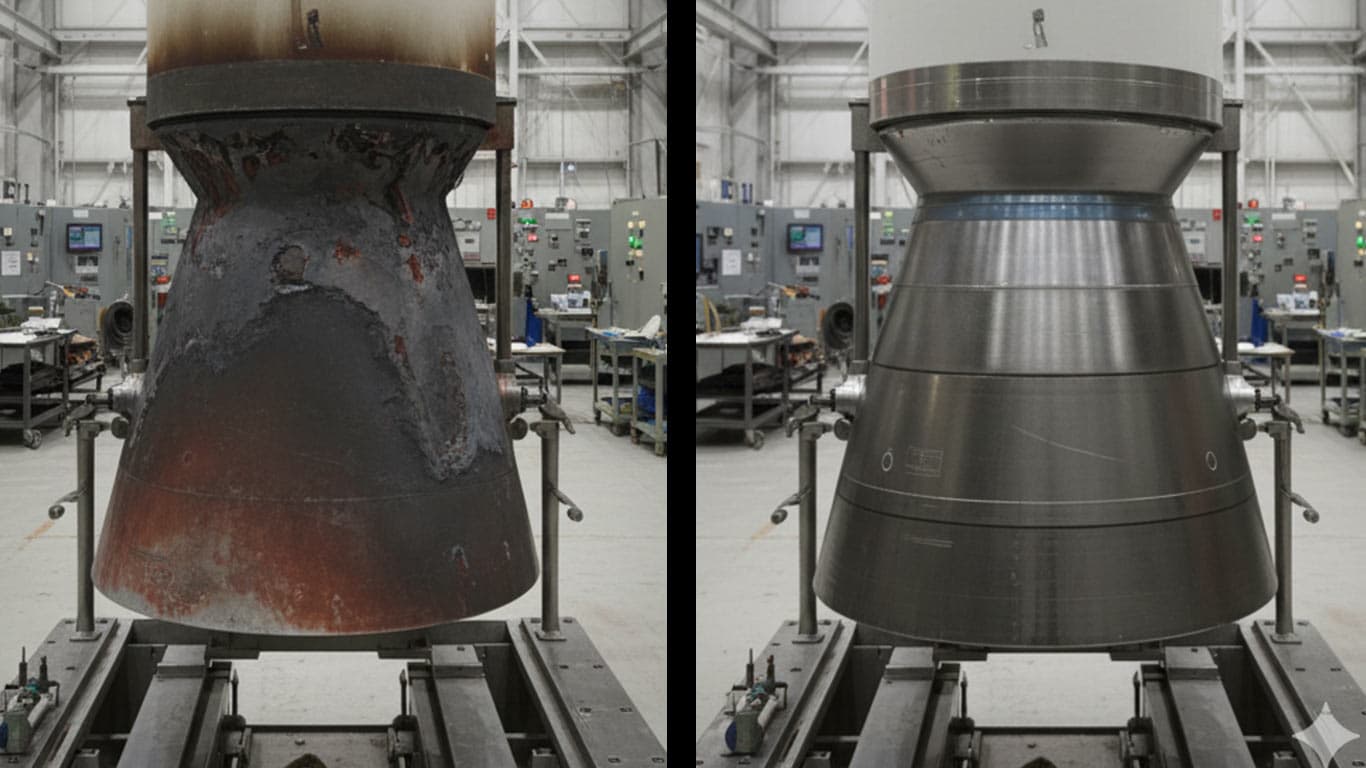
Titanium Carbide
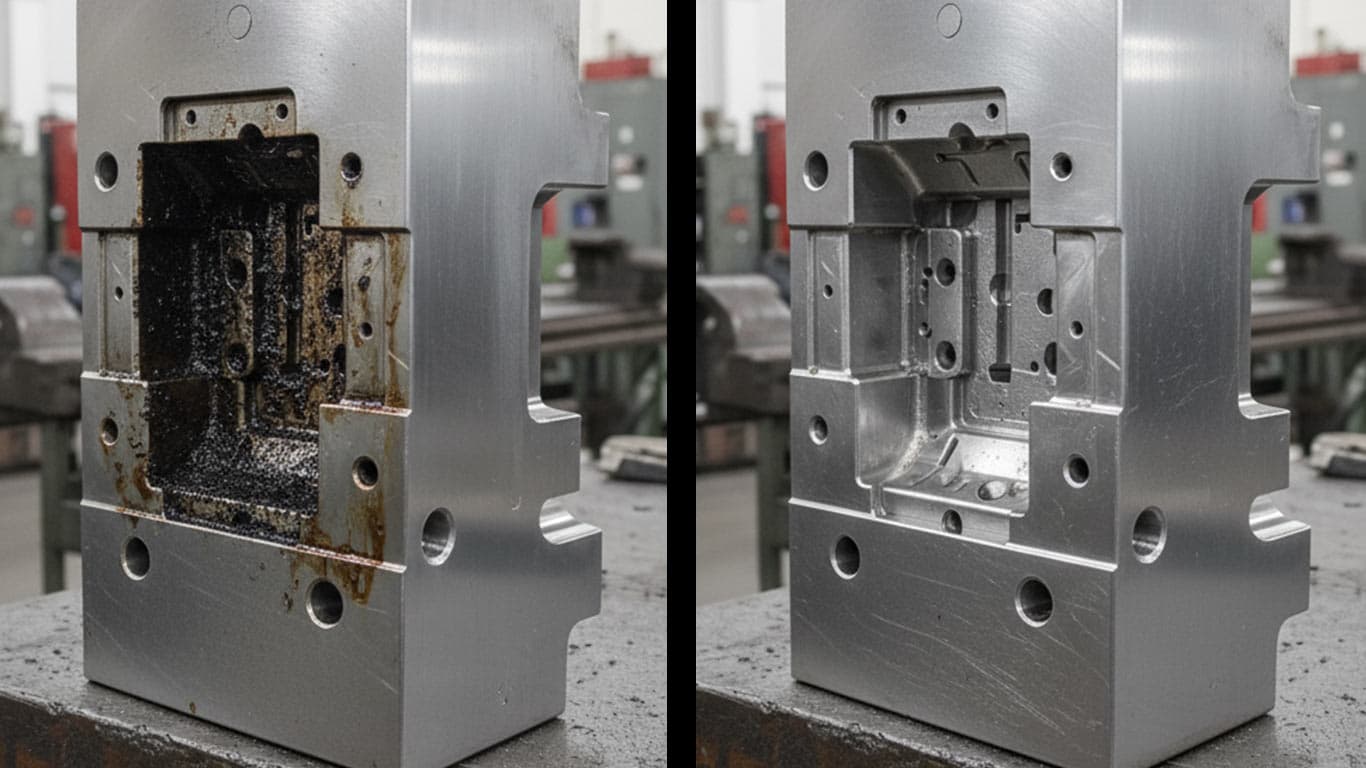
Tool Steel

Zinc
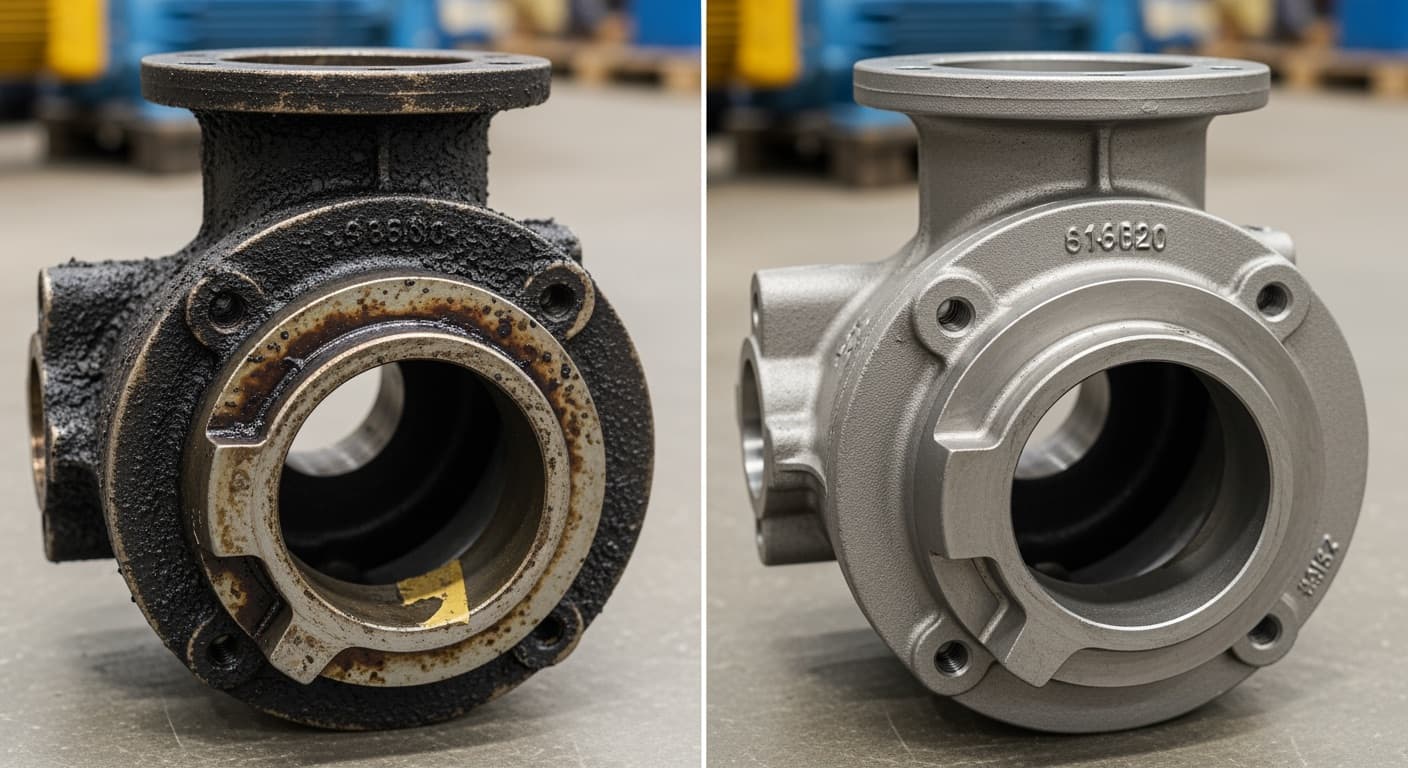
Titanium Alloy (Ti-6Al-4V)

Stainless Steel 316
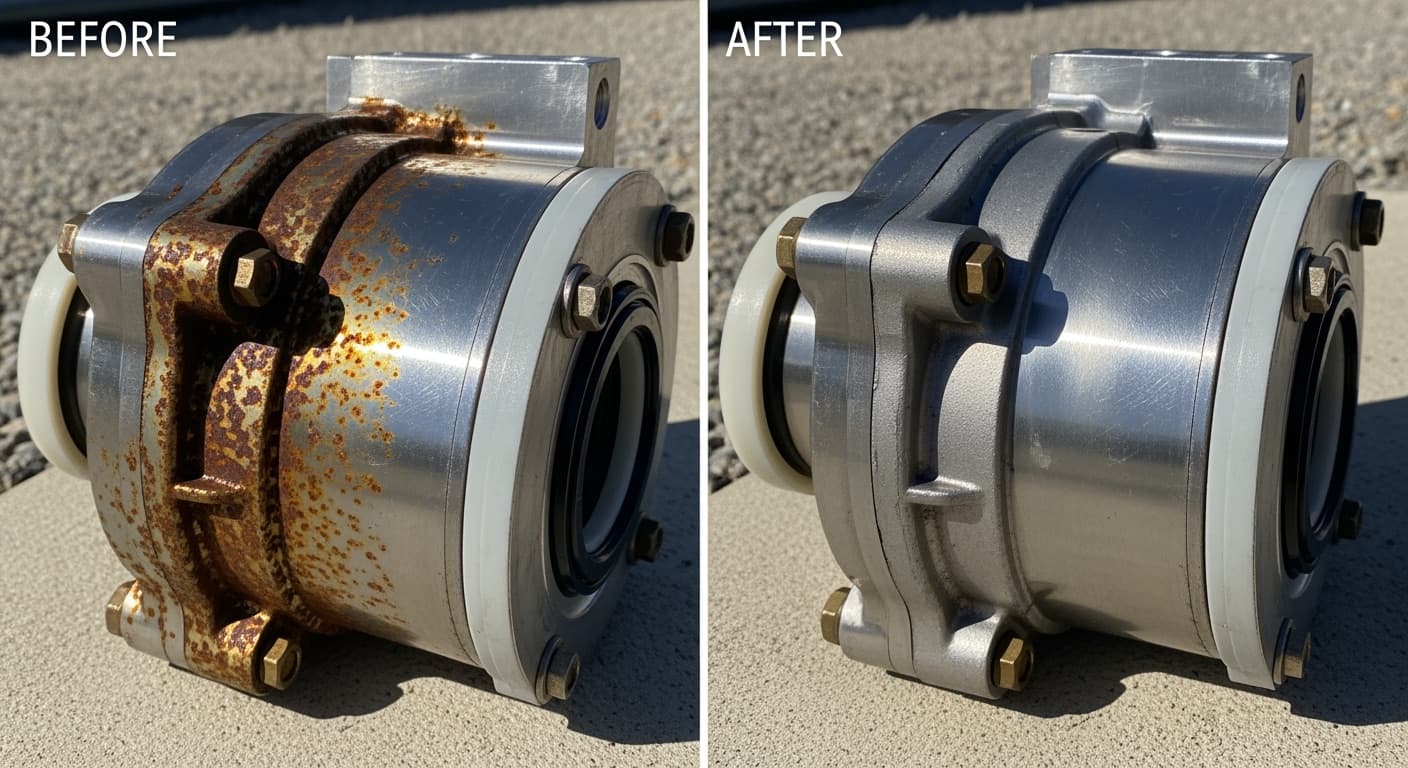
Stainless Steel 304

Aluminum Bronze

Aluminum Nitride
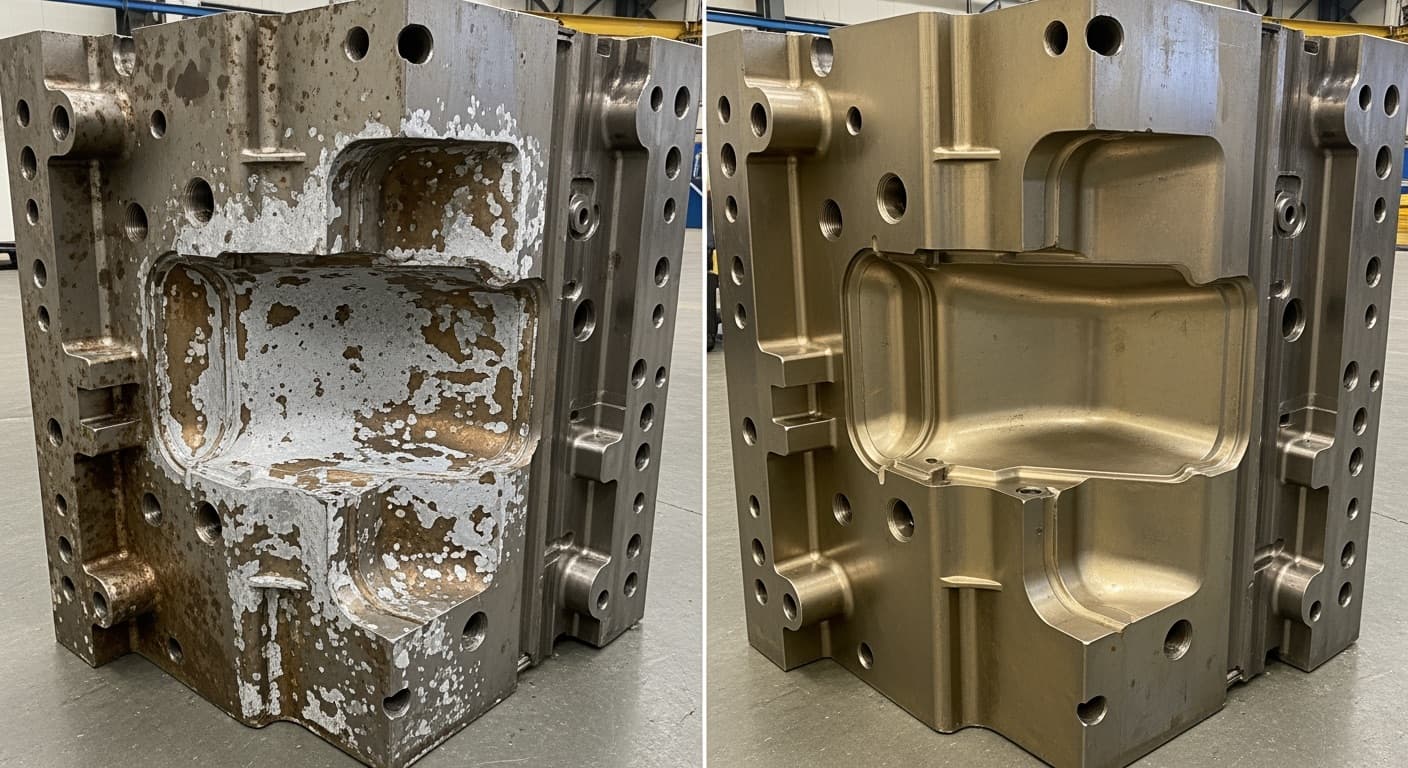
Titanium Nitride
Pharmaceutical Drug Residue Dataset
License: Creative Commons BY 4.0 • Free to use with attribution •Learn more




