


Yi-Chun LinPh.D.Taiwan
Laser Materials ProcessingPublished
Jan 6, 2026
Hard Water Mineral Scale
Mineral deposits contaminate surfaces unevenly across regions, forming thick layers on metals while staying thin on stones, and this difference affects cleaning outcomes. After exposure to moisture, buildup hardens quickly, so laser cleaning faces challenges in avoiding thermal damage. Contamination exhibits sticky patterns during formation, especially in humid areas. Treatment removes layers effectively on durable materials, but softer ones show cracking risks. Process achieves better uniformity on heat-resistant substrates.
Ikmanda Roswati, Ph.D. from Indonesia
Produced Compounds
Hazardous compounds produced during laser cleaning
Affected Materials
Materials where this contaminant commonly appears

Aluminum

Borosilicate Glass
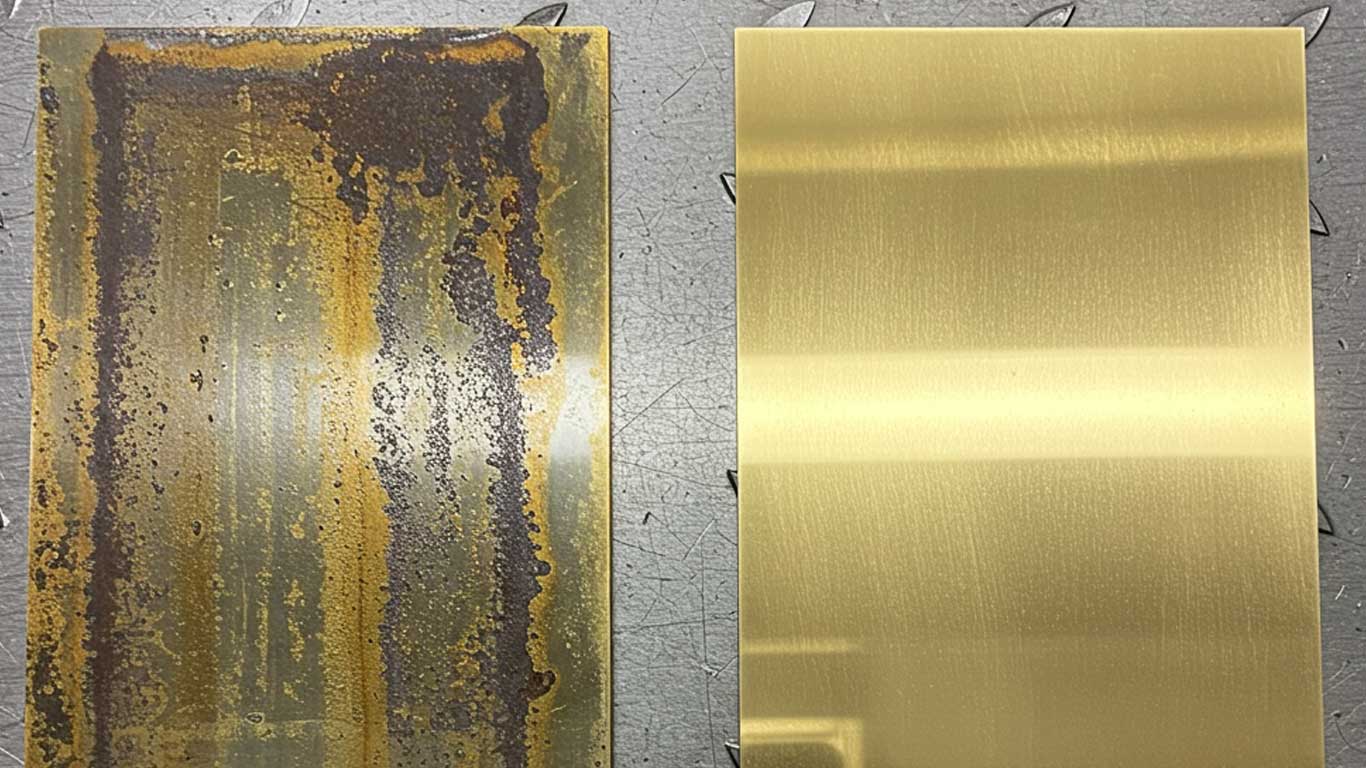
Brass

Bronze

Cast Iron

Concrete

Copper

Crown Glass

Fiberglass

Float Glass

Glass Fiber Reinforced Polymers GFRP
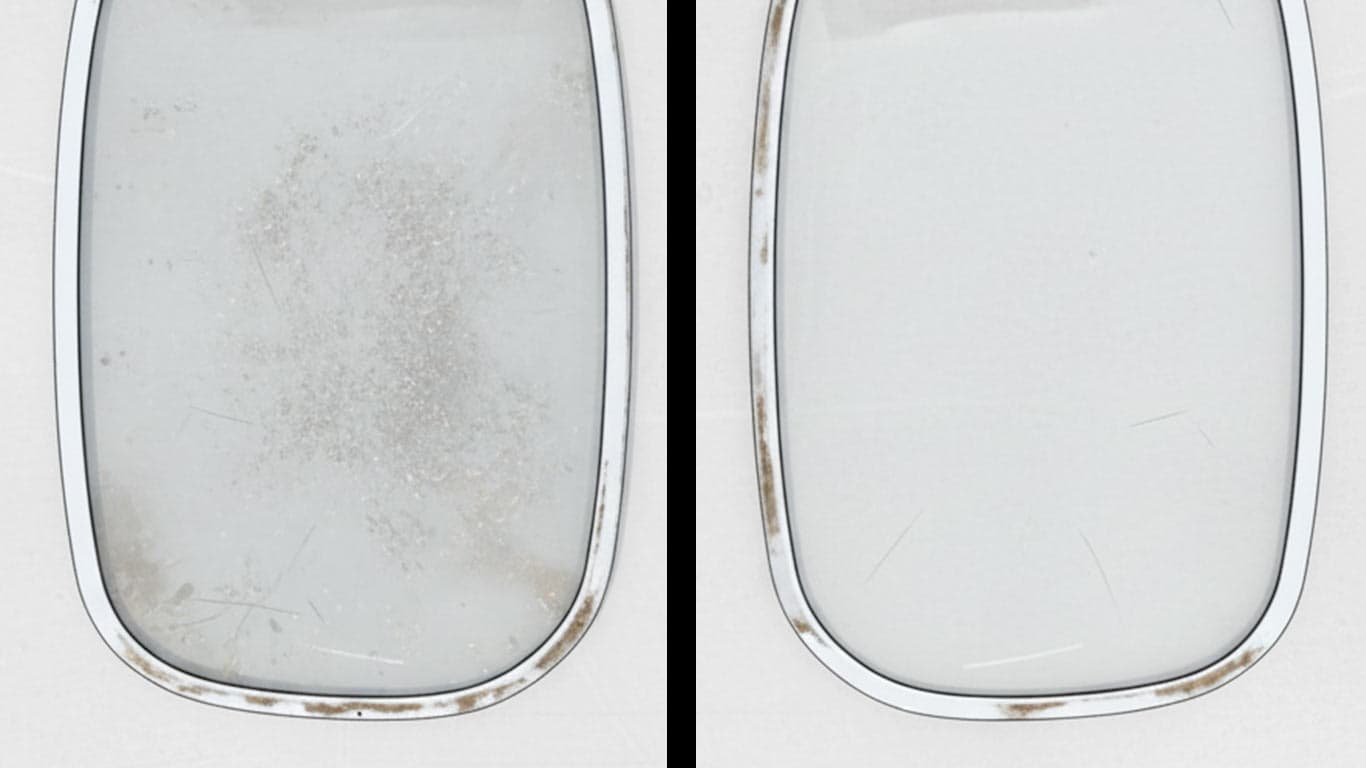
Gorilla Glass

Granite

Iron

Lead Crystal

Limestone

Magnesium

Marble

Nickel

Quartz Glass

Sandstone

Sapphire Glass

Slate

Soda-Lime Glass

Stainless Steel

Steel

Tempered Glass

Titanium
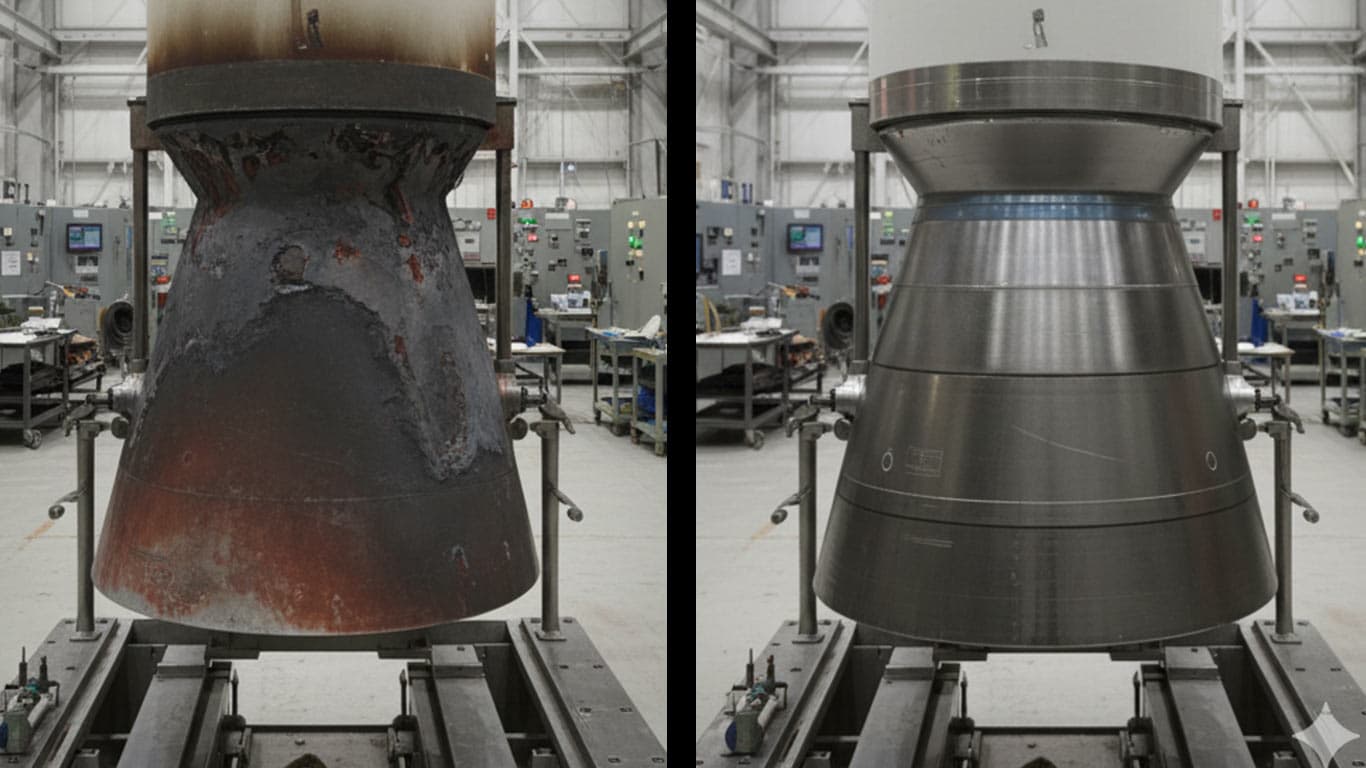
Titanium Carbide
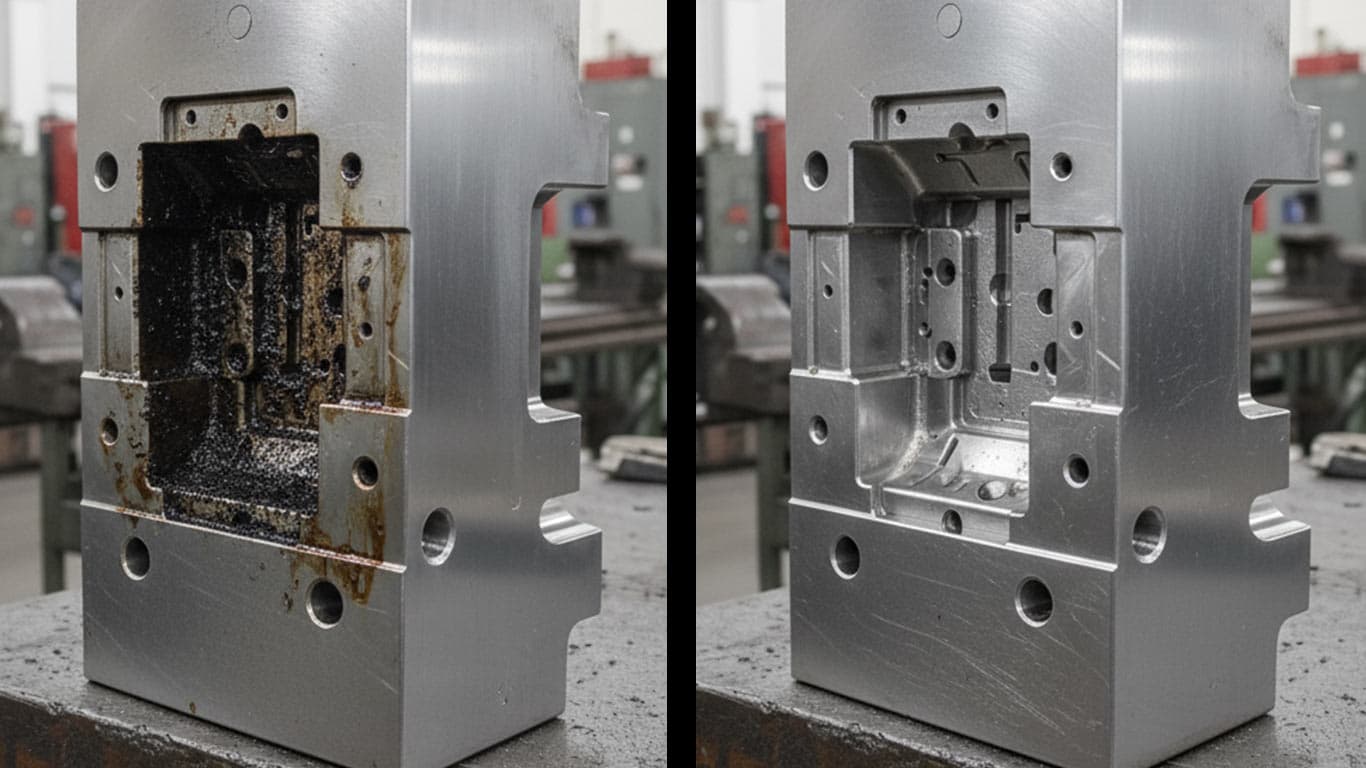
Tool Steel

Zinc

Aluminosilicate Glass
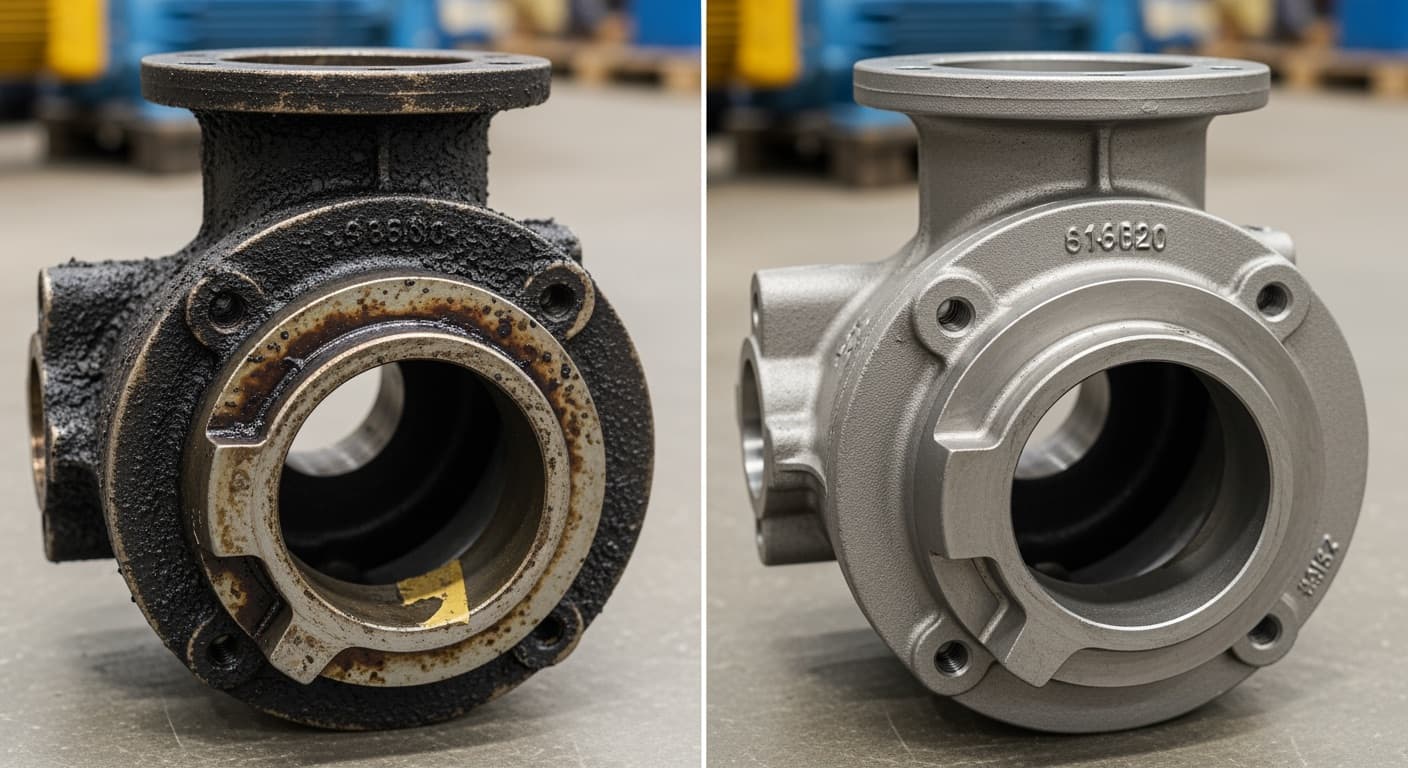
Titanium Alloy (Ti-6Al-4V)

Stainless Steel 316
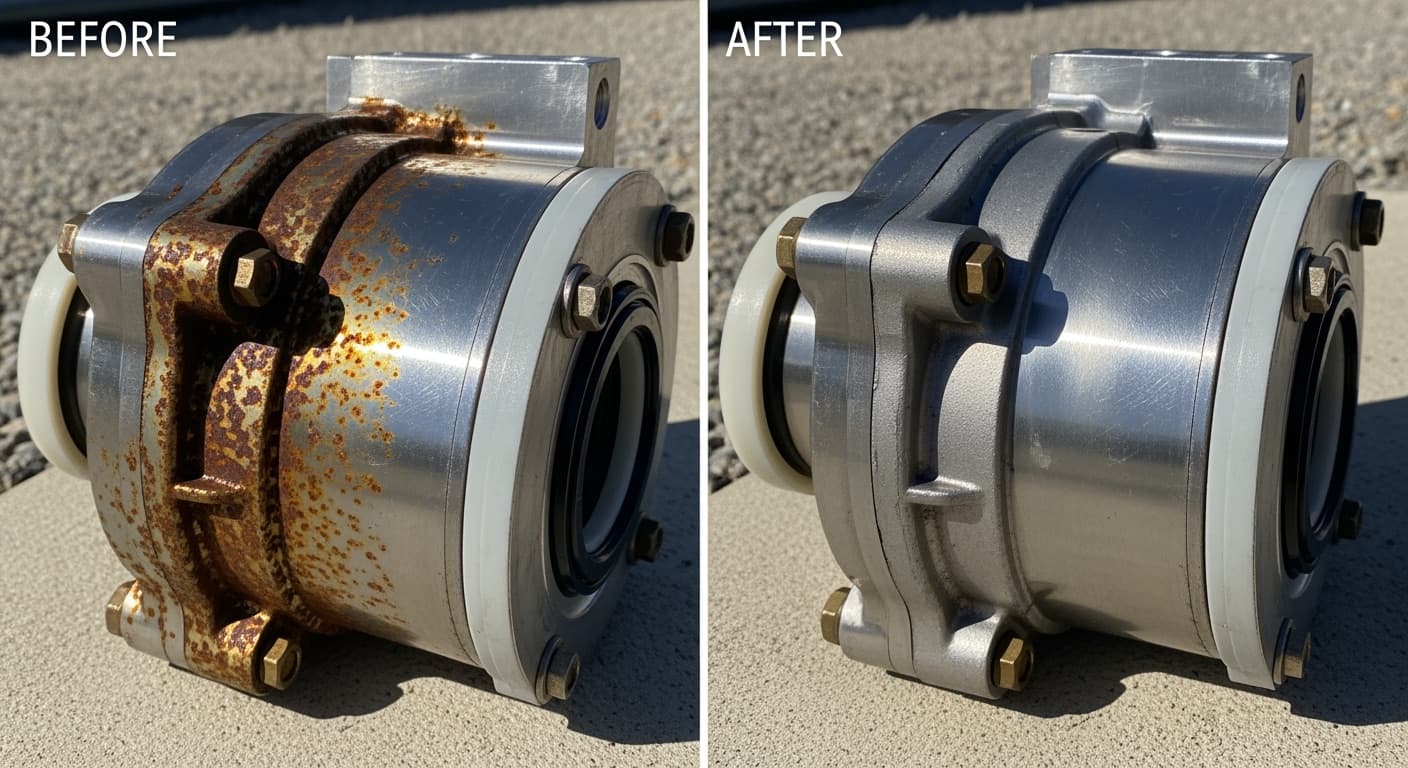
Stainless Steel 304

Aluminum Bronze

Aluminum Nitride
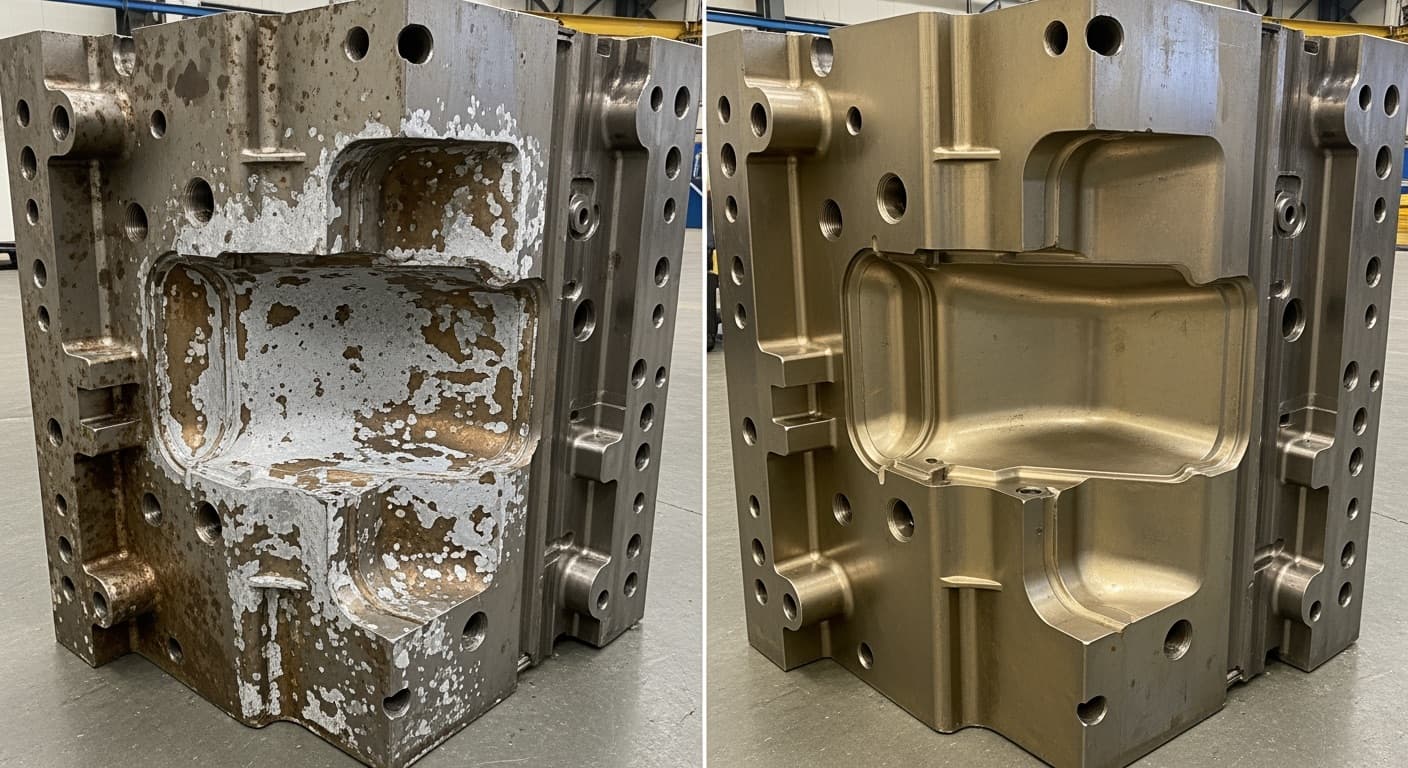
Titanium Nitride
Hard Water Mineral Scale Dataset
Download Hard Water Mineral Scale properties, specifications, and parameters in machine-readable formats
0
Variables
0
Safety Data
9
Characteristics
3
References
3
Formats
License: Creative Commons BY 4.0 • Free to use with attribution •Learn more
Get Started
Schedule a service or reach out for more information


